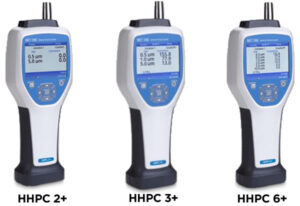
- Performance- ISO 21501-4 unit-to-unit accuracy & reproducibility
- Industry Standards- ISO 21501-4 , ISO 14644-1:2015, FCC, CE
- Light Source- Long Life Laser™ Diode
- Particle Size Analysis Range- 0.50|10.00|μm / 0.5|10|μm
- Coincidence Loss- 10% at 20,000,000 particles/m3
- Sample Volume- 10.00|µm
- Throughput- 28.32 L per minute
- Zero Count- ISO 21501-4 & JIS B 9921 (1 count or less in 5 min; 95% UCL)
- Count Alarms Display & Cycles- User-defined alarm counts displayed via LED on box or remote LED
- Item Specifications Referenced- 2088615-PF-EA
Facility Monitoring System
Item#: FMSFacility Monitoring Systems (FMS) from Beckman Coulter can be configured to meet a wide spectrum of cleanroom management needs for various industries—including pharmaceutical production. Among parameters that can be efficiently and reliably monitored using a Beckman Coulter FMS are non-viable/viable air sampling, differential pressure, air temperature and relative humidity. Each instrument is built around proven MET ONE technology, including the portable MET ONE 3400 series for routine monitoring and cleanroom classification, as well as the 6000 and 7000 series of fixed, non-viable air particle counters. MET ONE software platforms feature intuitive interfaces to help make them easy to use, thereby reducing the potential for human error that can occur with manual SOP monitoring. To avoid losses from product contamination, stack lights, audible alarms and process shutdown signals from a Beckman Coulter FMS can prompt an immediate production line response whenever an excursion happens. In addition, to help centralize reporting and simplify EUGMP/FDA compliance (e.g., for ISO 14644 and 21 CFR Part 11), Beckman FMS data can be integrated into a single-release, electronically signed paperless report.
Capabilities
- Project Management
- System Definition and Design
- Software Configuration
- System Installation
- System Validation
Service Options
- Onsite particle counter installation, software configuration, and system validation
- Regional Service Professionals provide onsite ISO 21501-4 calibration and service
- Service agreement options for pre-scheduled onsite calibration and maintenance (including discounts for repairs)
Data Management
- Collapse multiple monitoring systems into single integrated systems for easier validation, maintenance, and data management
- Industry standard interfaces (OPC, ODBC) for integration with other systems
- Redundant data management throughout the system eliminates single points of failure
Compliance
- ISO 14644 Cleanroom Management
- ISO 21501-4 Air Particle Counter Calibration for Accuracy and Repeatability across sensors
- 21 CFR Part 11 for management of electronic data records
- EU GMP Annex 1 for manufacture of sterile medicinal products